The AFM-IR lab at the Institute of Physical Chemistry, Paris-Saclay University (Orsay, France) is the pioneering research group in Infrared-Atomic Force Microscopy (AFM-IR). Founded by Alexandre Dazzi, the inventor of the AFM-IR technique, and now led by Ariane Deniset-Besseau, the group specializes in AFM-IR instrumental and methodological developments while continuously broadening the technique’s field of applications. In particular, the team specializes in the characterization of complex materials, ranging from astro to biosciences, including studies on pathological calcifications such as breast microcalcifications (MCs), described here for Surface Newsletter by Margaux Petay, former AFM-IR lab PhD student.

A Multimodal strategy to explore Breast Microcalcifications
Breast microcalcifications (MCs) are calcium-based deposits found in breast tissue, ranging in size from hundreds of nanometers to millimeters. These inorganic deposits can be present in both cancerous and benign breast lesions, yet their formation mechanisms remain poorly understood.
Over the past decade, Raman, IR and O-PTIR spectroscopy have been increasingly used to study the chemical composition of MCs in breast biopsies. However, these techniques are limited to a spatial resolution of about a micron at best (Raman and O-PTIR), which restricts the chemical imaging of individual MCs – analyses that could give new insights into their formation processes.
To address these limitations, we developed a multimodal and multiscale strategy that integrates SEM, μFTIR and AFM-IR, enabling a comprehensive morphological and chemical characterization of MCs from the micro to the nanoscale level directly within breast biopsy samples (Fig.1). This approach involves the following sequential steps:
- SEM: acquisition of micrographs at low, intermediate and high magnification. Localization of MCs within the tissue and morphological characterization at the micro and nanoscale
- μFTIR: acquisition of hyperspectral images. Analysis of the chemical diversity among MCs across the tissue
- AFM-IR: acquisition of topographical images, chemical maps at one wavenumber and hyperspectral images. High-resolution chemical imaging of individual MCs.
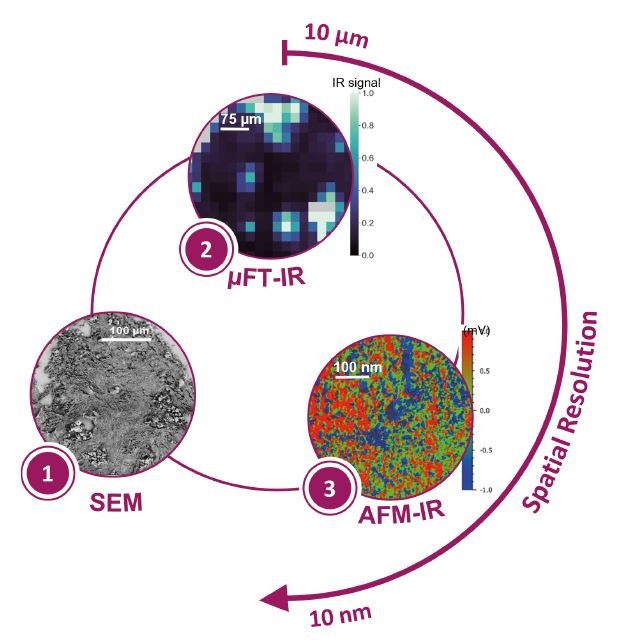
Fig. 1 Summarized workflow of the multimodal and multiscale approach.
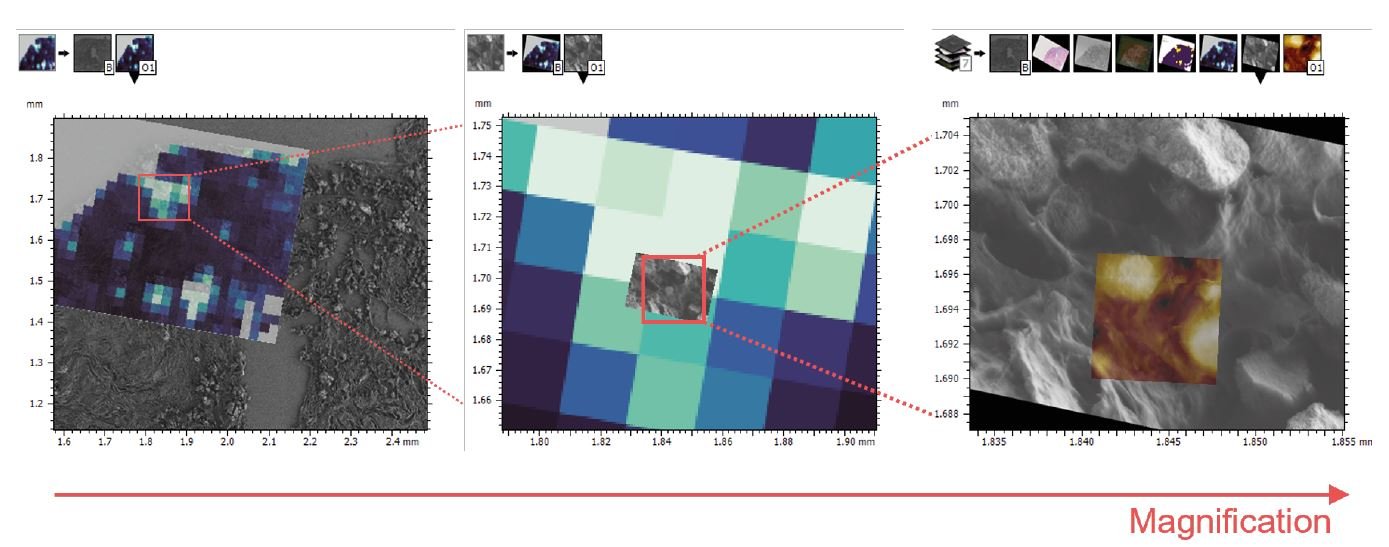
Fig. 2 μFT-IR and AFM-IR colocalization using SEM micrographs as reference images. From left to right: SEM cliché & μFT-IR 1655 cm-1 chemical map; μFT-IR & zoomed SEM micrograph and SEM micrograph overlay with AFM-IR topography.
Colocalizing multiscale chemical and structural information
To accurately compare MC physicochemical properties across different scales – from the microscale to the nanoscale – a registration step is essential to align SEM, μFT-IR and AFM-IR measurements within the same spatial coordinate system. This process can be challenging due to significant differences in the spatial resolution of these techniques.
To achieve this, we used SEM micrographs at low, intermediate and high magnifications as reference images. These images were then employed to register the μFT-IR and AFM-IR datasets using the Mountains® software colocalization tool with manual point positioning. This approach ultimately enabled the creation of a multichannel study with colocalized SEM micrographs, μFT-IR chemical map and AFM-IR measurements (topography and chemical maps) (Fig. 3).
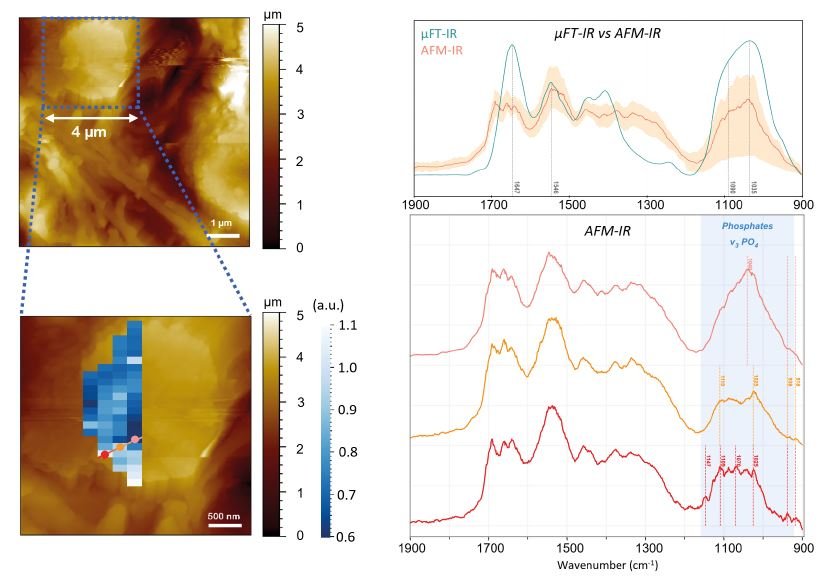
Fig. 3 AFM-IR analysis of a 4μm diameter MC. The chemical map overlaid with the topography corresponds to hyperspectral AFM-IR imaging showing 1109 cm-1 / 1035 cm-1 ratio, highlighting chemical heterogeneities within the MC’s calcium phosphate lattice. Red, orange and pink dots locate positions where local AFM-IR spectra (right panel) were acquired.
Unveiling microcalcification heterogeneities at the nanoscale
This multiscale approach offers the advantage of capturing the chemical diversity of MCs across the biopsy thanks to FTIR microscopy, while AFM-IR investigates individual MCs, particularly those too small to be accurately identified and characterized using standard IR microscopy, as shown in Figure 3, with a 4-micron diameter MC.
This approach provides a global and detailed chemical description of MCs within breast biopsies, delivering a holistic view of calcifications that was not previously achieved. If applied to a broader cohort of samples, this strategy could enhance our understanding of the mechanisms driving biomineralization in breast tissue.
Instruments & software used
Scanning electron microscope, μFT-IR, AFM-IR & Mountains® software.
Read more
Multimodal and multiscale analysis of complex biomaterials: optimization and constraints of infrared nanospectroscopy measurements, Petay M., Université Paris-Saclay, 2023, theses.hal.science/tel-04416918.
Multiscale approach to provide a better physicochemical description of women breast microcalcifications, Petay et al., C. R. Chim, 2022, doi.org/10.5802/crchim.210.